CNAs certification: flow chart of inspection organization accreditation and FAQ
Date:2021-07-30 14:32:00 Views:6817
catalog:
| What is recognition? | Basis of approval | Approved category |
| The nature of recognition | Role of recognition | Recognized characteristics |
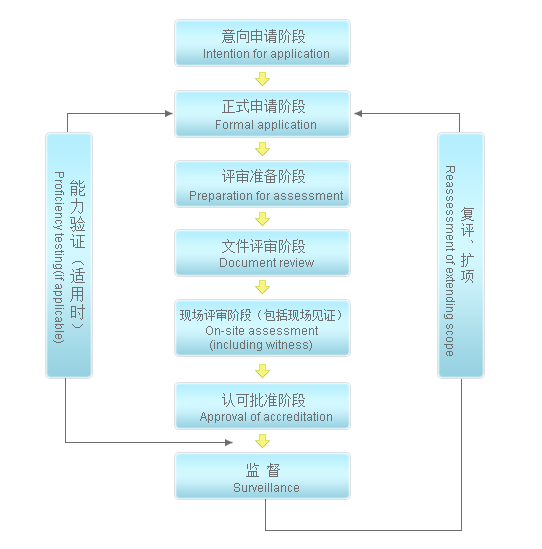
Flow chart for approval of inspection organization
Frequently asked questions about accreditation of inspection institutions
Q:What is recognition?
A: Accreditation is a third-party certificate formally indicating that the conformity assessment institution has the ability to carry out specific conformity assessment. Generally speaking, accreditation means that the accreditation institution reviews the conformity assessment institutions engaged in certification, testing and inspection in accordance with relevant international or national standards, confirms that they meet the requirements of relevant standards, further proves that they have the technical and management ability to engage in certification, testing and inspection, and issues accreditation certificates.
Q:What is the basis for recognition?
A: CNAs implements accreditation activities in accordance with standards, guidelines and other normative documents issued by international organizations such as ISO / IEC, IAF, ILAC and APAC, as well as accreditation rules, guidelines and other documents issued by CNAs. The accreditation rules specify the policies and procedures for CNAs to implement accreditation activities; Accreditation criteria are the requirements that CNAs accredited conformity assessment institutions should meet; The approval guide is the description or application guide of the approval criteria. CNAs conducts conformity review on the management and technical capabilities of certification bodies, laboratories and inspection institutions in accordance with the provisions of accreditation specifications.
Accreditation criteria are the basic basis for accreditation review, which specifies the basic requirements to be met by conformity assessment institutions such as certification institutions, laboratories and inspection institutions. The basic criteria for CNAs accreditation activities mainly include: ISO / IEC17021 requirements for conformity assessment management system audit and certification bodies, ISO / iec17065 requirements for conformity assessment product, process and service certification bodies, ISO / IEC17024 General requirements for conformity assessment personnel certification bodies, ISO / IEC17025 General requirements for testing and calibration laboratory capabilities ISO / iec17020 operational requirements for various inspection institutions for conformity assessment, ISO15189 special requirements for the quality and competence of medical laboratories, iso17034 General requirements for the competence of producers of reference materials / standard samples and ISO / iec17043 General requirements for the verification of conformity assessment competence. If necessary, CNAs also formulates application guidelines or application instructions on the basis of basic recognition criteria for specific situations in some industries or technical fields.
Q:What are the recognized categories?
A: Generally, according to the classification of recognition objects, recognition is divided into certification body recognition, laboratory and related institutions recognition and inspection institutions recognition.
1、 Approved by certification authority
Accreditation by certification body means that the accreditation body shall, in accordance with laws and regulations and based on the requirements of GB / t27011, respectively:
1) The national standard GB / t27021 requirements for audit and certification bodies of conformity assessment management systems (equivalent to the international standard ISO / IEC17021) shall be used as the criterion to review the management system certification bodies and confirm whether they have the ability to carry out management system certification activities;
2) The national standard GB / t27065 General requirements for product, process and service certification bodies (equivalent to the international standard ISO / iec17065) shall be used as the criterion to review the product certification bodies and confirm whether they have the ability to carry out product, process or service certification activities;
3) The national standard GB / t27024 General requirements for personnel certification bodies for conformity assessment (equivalent to the international standard ISO / IEC17024) is used as the criterion to review the personnel certification bodies and confirm whether they have the ability to carry out personnel certification activities.
The accreditation body shall formally recognize the certification body that meets the requirements and issue an accreditation certificate to prove that the certification body has the technical and management ability to implement specific certification activities.
2、 Recognized by laboratories and relevant institutions.
Laboratory accreditation means that the accreditation institution shall, in accordance with laws and regulations and based on the requirements of GB / t27011, respectively:
1) The national standard GB / t27025 General requirements for the competence of testing and calibration laboratories (equivalent to the international standard ISO / IEC17025) shall be used as the criterion to review the testing or calibration laboratory to confirm whether it has the ability to carry out testing or calibration activities;
2) The national standard GB / t22576 special requirements for the quality and competence of medical laboratories (equivalent to the international standard ISO15189) is used as the criterion to review the medical laboratory and confirm whether it has the ability to carry out medical testing activities;
3) According to the national standard gb19489 General requirements for laboratory biosafety, the pathogenic microorganism laboratory was reviewed, and it was confirmed that the biosafety protection level of the laboratory reached the corresponding level;
4) The national standard GB / t27043 General requirements for conformity assessment and capability verification (equivalent to the international standard ISO / iec17043) shall be used as the criterion to review the capability verification plan providers to confirm whether they have the capability to provide capability verification;
5) National standard GB / t15000 7. Based on the general requirements for the production capacity of reference materials / standard samples (equivalent to the international standard iso17034), review the reference material producers to confirm whether they have the production capacity of reference materials.
The accreditation institution shall formally recognize the conformity assessment institution that meets the requirements and issue an accreditation certificate to prove that the institution has the technical and management ability to implement specific conformity assessment activities.
3、 Approval of inspection organization
Inspection institution accreditation refers to the accreditation institution reviewing the inspection institution in accordance with laws and regulations, based on the requirements of GB / t27011 and based on the national standard GB / t27020 operational requirements of various inspection institutions for conformity assessment (equivalent to the international standard ISO / iec17020) to confirm whether it has the ability to carry out inspection activities.
The accreditation institution shall formally recognize the inspection institution that meets the requirements and issue an accreditation certificate to prove that the inspection institution has the technical and management ability to carry out specific inspection activities.
Q:What is the essence of recognition?
A: By being recognized by the accreditation body, the conformity assessment institution proves that it has the ability to provide specific conformity assessment services within the approved scope according to the specified requirements, which is conducive to promoting its conformity assessment results to be widely believed, accepted and used by the society and trade.
Recognition is to review the specific capabilities (including technical and management capabilities) of the conformity assessment institution applying for recognition based on good faith and relevant standards or normative documents, and to confirm that the institution has the ability to carry out conformity assessment activities and issue conformity assessment certificates or reports in accordance with the specified requirements, The conformity assessment certificate or report issued in accordance with the specified procedures within its recognized scope shall be credible. The accreditation institution shall investigate and deal with the acts and results found or received by the relevant conformity assessment institution that do not meet the accreditation requirements in accordance with the specified procedures and requirements. If the circumstances are serious, the corresponding accreditation qualification shall be suspended or revoked. Accreditation is based on the confirmation of sampling. The accreditation body does not approve each conformity assessment certificate or report issued by the conformity assessment body.
The conformity assessment institution shall operate in accordance with the requirements of accreditation, ensure that the ability and impartiality of its accredited conformity assessment activities meet the accreditation requirements and continue to be maintained, and be responsible for the authenticity, accuracy and effectiveness of the issued certification, testing or inspection certificates or reports. Through the recognized conformity assessment organization, even if there are non-conforming conformity assessment certificates or reports, the causes of non-compliance can be found under the standardized operation system, so as to make the conformity assessment activities more traceable, and timely take corrective and preventive measures to continuously improve the ability and improve the service quality.
Q:What is the role of recognition?
A: -- in terms of capability evaluation: confirm that the conformity assessment organization has the ability to implement specific conformity assessment.
-- in terms of government supervision: enhance the government's confidence in the use of certification, testing and inspection and other conformity assessment results, reduce the uncertainty of making relevant decisions and the technical evaluation link in administrative licensing, and reduce the risk and cost of administrative supervision.
-- in terms of promoting trade: promote international mutual recognition of conformity assessment results and foreign trade by signing multilateral or bilateral mutual recognition agreements with international organizations, regional organizations or foreign accreditation institutions.
- in non trade areas: promote the improvement of standardization, quality and capacity in non trade areas such as health, safety and social services.
-- in terms of market competition: help conformity assessment institutions and their customers enhance social popularity and market competitiveness.
-- in terms of continuous improvement: through systematic and standardized technical evaluation and continuous supervision of conformity assessment institutions, it is helpful for conformity assessment institutions and their customers to achieve self-improvement and self-improvement.
Q:What are the characteristics of recognition?
A: Authority, independence, impartiality, technicality, standardization, unity and Internationality




 Weixin Service
Weixin Service

 DouYin
DouYin
 KuaiShou
KuaiShou