Teach you the whole process of applying for laboratory accreditation
Date:2021-05-26 18:08:00
Views:3905
How to apply for laboratory accreditation is often consulted online? For this problem, we have sorted out the contents released according to China Accreditation. Starting from the application for account number, we will teach you to apply for laboratory accreditation. The contents are for reference only.
The first step is to apply for an account
(1) Account number acquisition method of unauthorized institutions
a) Visit CNAs website: www.cnas.com org. Cn, "home page - online service - online application for laboratory / inspection institution accreditation business - enter the system", open the system login page.
b) Click the "new institution user registration" link at the bottom of the login page to pre apply.
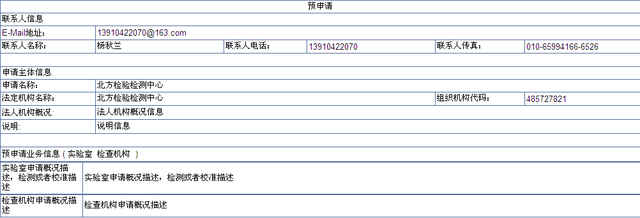
c) At the top of the pre application page are the precautions and pre application operation steps, which should be read carefully before operation. The following is the information that needs to be input by institutional users, including contact information, application subject information and pre application business information.
matters needing attention:
1. The pre application for registration is only for institutions that have never applied for accreditation. If you have obtained an accreditation certificate (regardless of the current status of the certificate), please obtain it through the "forget password" function in the lower right corner of the login page, or contact the No. 7 accreditation Office of China National Accreditation Commission for conformity assessment (hereinafter referred to as "CNAs") to obtain the login account and password of the system institution.
2. The submitted pre application can be found in the email filled in the pre application to view and verify the email.
3. After passing the pre application, you can apply online, but at the same time, you still need to send the paper application to CNAs 7 for application.
4. If an approved institution has to apply for other qualifications. Please log in to the system first, and then change the business field to add other qualifications.
For example, it has obtained the laboratory qualification, but now it has to obtain the qualification of other inspection institutions.
5. The contact information is very important, which affects the contact with the pre application acceptance personnel and the email sent by the receiving system in the future.
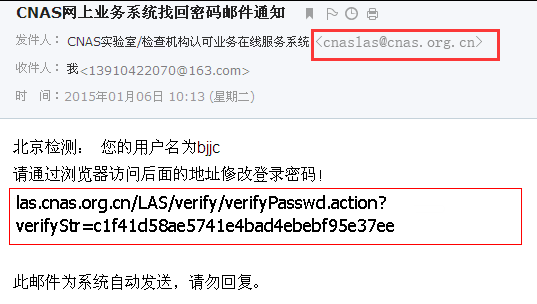
6. At present, the system mailbox used by the system to send mail to users is cnaslas@cnas.org.cn 。
7. Pre application business information is very important. Select the corresponding business and fill in the corresponding description information. You should select at least one item and fill in the description of selecting this field in the input box corresponding to the option.
8. For organization code, please remove the "-" in the middle when filling in. For example, xxxxxxxx-l is directly filled in as xxxxxxxl.
9. The items marked with red asterisk in the page are required. If they are omitted, the system will not be able to save the filled information.
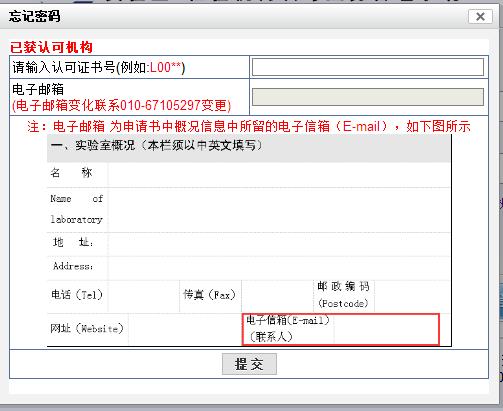
d) After filling in all the information, click the button, and the system will verify the correctness of the input information. After successful submission, the prompt information will be displayed as shown in the figure below:
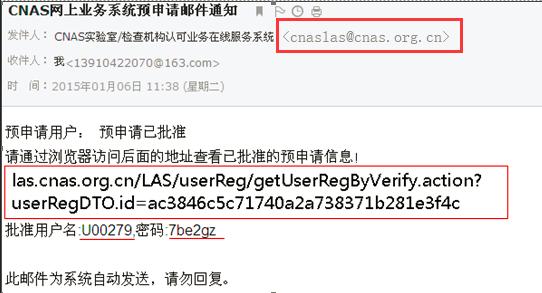
e) Institutional users log in to the mailbox, open the inbox and view the email notification:
f) Access the address of the verification pre application in the email through the browser, and open the web page as follows:
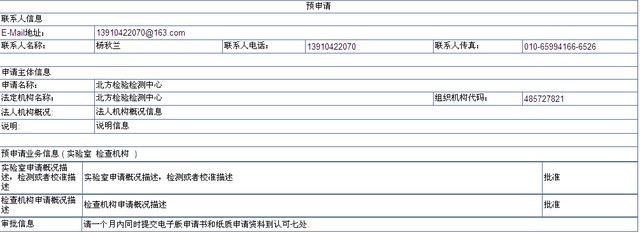
Visit the address to view the submitted pre application information through the browser, and open the web page as follows:
g) After the pre application is verified successfully, the pre application record will be transferred to the pre application acceptance list of CNAs. Users with the pre application management role of CNAs will process it and the conclusion is approved or disapproved.
If the approval organization pre applies, it will send an email to notify the organization user. The organization user logs in to the mailbox, opens the inbox, views the email notification, and obtains the approval user name and password. The English letters in the user name and password should be case sensitive.
Institutional users enter the user name and password obtained after pre application approval to log in to the system, and click the menu item "maintain user information" - "modify user account password" to modify the password.
h) Access the address in the email to view the approved pre application information through the browser, and open the web page as follows:
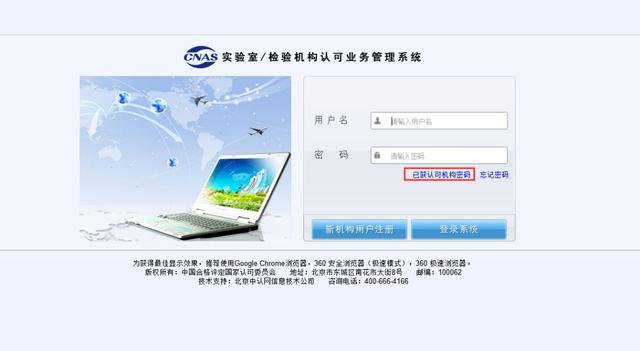
(2) Account number acquisition method of authorized institution:
a) Visit CNAs website: www.cnas.com org. Cn, "home page - online service - online application for laboratory / inspection institution accreditation business - enter the system", open the system login page.
b) After entering the user login page, click "authorized institution password" to retrieve the institution account password.
c) Enter the certificate number, and the system will automatically bring out the contact email corresponding to the previous application for approval, and click Submit. If you need to modify your email, please send an email to tianss@cnas.org.cn The information includes: company name, approval registration number, original email address, changed email address, contact name and telephone number. Please log in to the system again 1-2 working days after sending the email, and use the changed email to retrieve the original user name and password. If you have successfully modified your email and still can't log in, please send an email to kuiy@cnas.org.cn After consultation, relevant staff will reply by email within 2-3 working days.
d) Then the system will send an email to your email address, and then open the email for password change.
e) Access the web address in the email through the browser and open the institutional user login password modification page:
f) In the user's new password and confirm new password text boxes, enter the new password, click the button, and a successful prompt will pop up.
Click "OK" to open the system login page.
Step 2: complete online submission
(1) Log in to "laboratory / inspection institution accreditation business system" and fill in relevant information
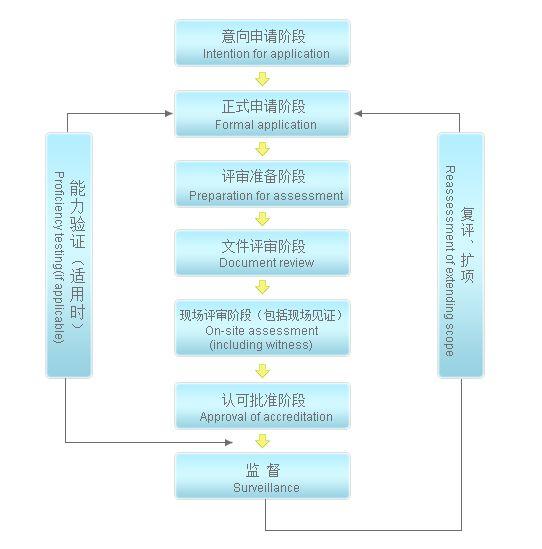
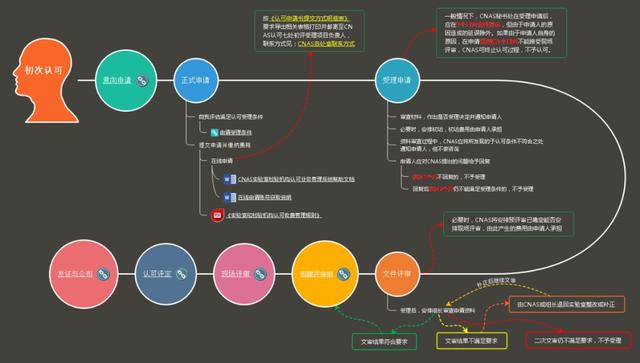
Refer to the following laboratory accreditation flow chart for specific steps:
Guide to Competence Accreditation of testing and calibration laboratories
This guide is applicable to the whole process of testing and calibration laboratories from accreditation application, on-site review to accreditation assessment and approval, as well as the main key points and precautions in the accreditation process. At the same time, this guide also includes the accreditation rules, guidelines and guidelines related to testing and calibration laboratories, as well as the directory and ability query of approved laboratories.
The most convenient way is to log in to the CNAs official website, directly download and use or open the client program online. The link is as follows (it is recommended to open and browse with a computer):
Introduction to Competence Accreditation of testing and calibration laboratories
(partial contents)
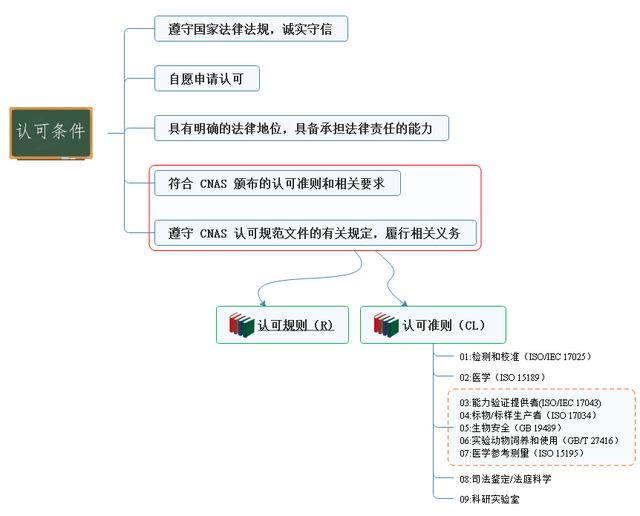
Approval conditions
Initial approval
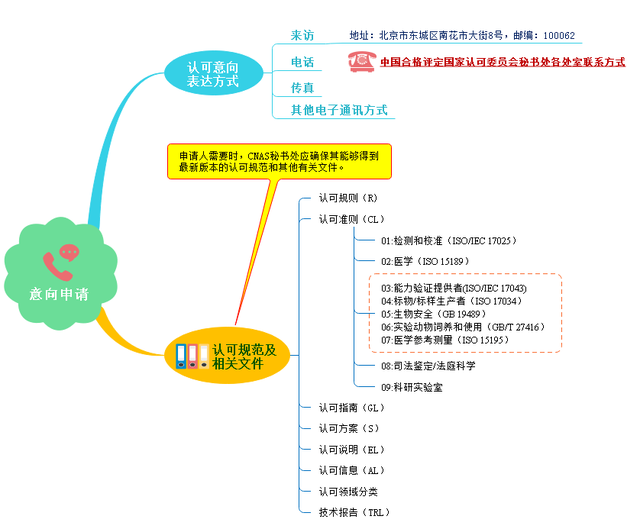
(1) Intention application
(2) Application acceptance conditions
According to cnas-rl01:2019 laboratory accreditation rules
(Note: the key marks below are the contents of 2019 edition different from 2018 edition RL01):
6 application acceptance requirements
6.1 the application materials submitted shall be true and reliable, and the applicant shall not commit fraud, conceal information or deliberately violate the recognition requirements.
Note: acts violating the authenticity and reliability of application materials include but are not limited to:
——The application materials are inconsistent with the facts;
——The application materials submitted are untrue;
——Self contradiction or time logic error in the same material or between materials;
——Similar to other applicants.
6.2 the applicant shall have a basic understanding of the relevant requirements of CNAs, and have conducted effective self-assessment. The application materials submitted are complete, accurate and clear.
Note: domestic laboratories applying for accreditation shall submit complete application materials in Chinese, and can provide Chinese and foreign comparison materials when necessary.
6.3 the applicant has a clear legal status and its activities shall meet the requirements of national laws and regulations.
6.4 the management system meeting the approval requirements has been established and has been officially and effectively operated for more than 6 months. That is, the management system covers all the application scope, meets the requirements of recognition criteria and its application instructions in special fields, and has operable documents. The organization setting is reasonable, the post responsibilities are clear, and the interfaces between documents at all levels are clear.
6.5 complete internal audit and management review have been conducted, and the expected purpose can be achieved, and all system elements shall have operation records.
Note: internal audit and management review shall be conducted 6 months after the operation of the management system.
6.6 the applied technical capability meets the requirements of cnas-rl02 capability verification rules.
6.7 the applicant has sufficient resources to carry out testing / calibration / identification activities within the scope of application, for example, key personnel, including authorized signatories, shall be able to meet relevant qualification requirements.
6.8 the quantity traceability of instruments and equipment used shall meet the relevant requirements of CNAs.
6.9 the technical capability applied for approval shall have corresponding testing / calibration / appraisal experience, and the above experience shall cover all items / parameters applied.
Note 1: the test / calibration / identification capability applied by the applicant shall be a frequently carried out and mature project.
Note 2: those who do not apply for the main business scope of the laboratory but only apply for the secondary work field will not be accepted in principle. In principle, those who have applied for the main business scope but do not apply for recognition of the main items but only apply for recognition of the secondary items will not be accepted.
Note 3: the applicant shall have sufficient and continuous testing / calibration / identification experience to support the ability applied for recognition. If there is no testing / calibration / appraisal experience in recent two years, in principle, the ability will not be accepted. For infrequent testing / calibration / identification activities, such as less than once a month, the applicant shall submit recent method validation and relevant quality control records at the time of approval application. For specific testing / calibration / identification items, if the applicant cannot establish quality control measures due to too few samples received and entrusted, in principle, this ability will not be accepted.
6.10 CNAs has the ability to test / calibrate / identify the applicant's application and carry out recognition activities.
6.11 CNAs approval criteria and requirements cannot be used as the applicant's ability to apply for approval.
6.12 other requirements deemed necessary by CNAs Secretariat.
6.13 the applicant's application for approval will not be accepted under the following circumstances:
a) The application materials submitted by the applicant are inconsistent with the facts, or the application materials submitted are untrue, or the applicant has fraud, concealment of information or intentional violation of recognition requirements.
b) The applicant cannot abide by the contents of the recognition contract on fairness, honesty, integrity and self-discipline.
c) It cannot meet the requirements of 6.2 ~ 6.12 above.
d) 5.1.2.4.
6.14 when CNAs decides not to accept the applicant's application and the applicant submits the approval application again, the following requirements shall be met according to different circumstances:
a) If the application for recognition is not accepted due to the reasons described in 6.13a) and b), the CNAs Secretariat will no longer accept the applicant's application within 36 months after making the acceptance decision. Before gaining confidence in the integrity, integrity and self-discipline of the laboratory, the re application for recognition will not be accepted.
b) If the applicant's management system fails to meet the recognition requirements or there are problems in the effectiveness of system operation, the application for recognition will not be accepted (if it fails to meet the requirements of articles 6.4 and 6.5), the applicant must submit the application for recognition again 6 months after making the acceptance decision.
c) If the application for approval is not accepted because the technical content cannot meet the requirements (e.g. articles 6.6 ~ 6.9), the applicant must meet the relevant technical requirements before submitting the application for approval again.









 Weixin Service
Weixin Service

 DouYin
DouYin
 KuaiShou
KuaiShou